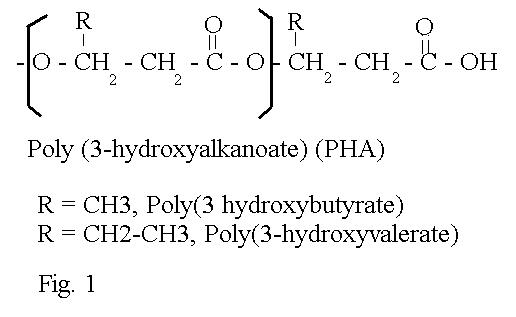
PHAs: Chemistry, History, and Biology
Chemistry of the PHAs
Of all the biodegradable plastics being studied, those that have generated the most interest are the poly(3-hydroxyalkanoates) (PHAs) which are made by bacteria. Like all plastics, PHAs are polymers, long molecules made up of many small subunits (monomers) which have been joined together. In the case of PHAs, the monomers are 3-hydroxyalkanoates. An alkanoate is simply a fatty acid which is a linear molecule containing just carbon and hydrogen (an alkane) with a carboxyl group at one end (making an alkanoate). Furthermore, these monomers have a hydroxyl group (OH) at the 3rd carbon (what used to be called the beta position), making these beta or 3-hydroxyalkanoates. The hydroxyl group of one monomer is attached to the carboxyl group of another by an ester bond; these plastics are thus polyesters.
As is shown in figure 1, the polyester linkage creates a molecule which has 3-carbon segments separated by oxygen atoms. The remainder of the monomer becomes a sidechain off the main backbone of the polymer. Most of the PHAs encountered in nature are poly(beta-hydroxybutyrate) (PHB), in which the monomer unit is hydroxybutyric acid and the side chain is a methyl group. Other monomer units occur in nature, and many others can be produced in the laboratory by feeding unusual carbon sources to bacteria. Most PHAs, even what we call PHB, are actually copolymers, and contain some amount of another type of monomer unit.
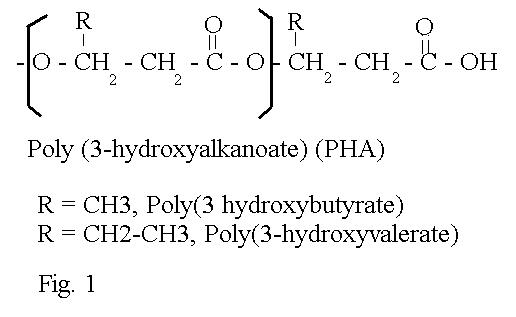
The composition of the PHA has a direct effect on the physical properties of the plastic, in part due to reasons that will be discussed below. PHB, with its short methyl sidechain, is a very crystalline and very brittle polymer. Industrially, it is difficult to use because the temperature at which it melts is very close to the temperature at which it begins to decompose. Its high degree of crystallinity causes it to crack easily. As a result, the PHA used commercially is PHBV, a copolymer of hydroxybutyrate and hydroxyvalerate (5 carbons long). PHBV is a random copolymer, meaning that the monomer units do not occur in the chain in any particular order. PHBV can still crystallize, but it produces a much more supple plastic and melts at a lower temperature, making processing easier. PHB and PHBV have properties similar to polypropylene, and bottles made from these polyesters feel just like "normal" plastic. The flexibility increases with sidechain length throughout the PHA family, largely because of a loss of crystallinity. Polymers composed mostly of hydroxyoctanoate, an 8- carbon monomer, are elastic. Longer sidechain polymers are so soft that they are gummy or glue-like. This remains one of the potential values of PHAs, that by feeding bacteria an appropriate substance, a PHA with specific desirable properties can be produced.
History of the PHAs
Biologists have known of the existence of PHAs since 1925 when the French scientist Lemoigne described the presence of lipid granules in the cytoplasm of bacterial cells. He went on to analyze these and found that they were polymers of hydroxybutyric acid (PHB). Microbiologists use the presence or absence of PHB as a taxonomic marker for the identification of bacteria. In the 1960s, researchers discovered that there were polyesters other than PHB, and the general term PHA for this family was coined. Since that time, scientists interested in novel plastics have become aware of the PHAs, and social and economic forces have supported research in the area.
The interest in PHAs would not have become so intense were it not for the fact that PHAs are natural materials, many of which are now known to be degraded by microorganisms. Thus PHAs are truly biodegradable plastics and are being considered more than a laboratory curiosity. Symposia on degradable materials in general and PHAs in particular have been held, a society for biodegradable polymers has been formed, and a journal has been launched. ICI, the British chemical conglomerate, used to sell PHBV as a plastic for containers to distributors in both Europe and the U.S. ICI sold the rights to the product, trade-marked Biopol, to the agricultural giant Monsanto in the late 1990s, and in 2001 the company Metabolix bought the rights.
Biology of the PHAs
When hearing for the first time that bacteria produce plastics, many persons wonder, "why?" PHAs are a storage form of carbon and energy for the bacteria that produce them. The analogy usually used is that of a bear storing up fat for hibernation. PHAs are synthesized when carbon is in excess, but some other nutrient needed for growth is missing. This nutrient is often a nitrogen source, but it could be oxygen (for a strict aerobe) or some other essential element. When the missing nutrient becomes available, the PHA is broken down by the cell and the carbon is used for energy or biosynthesis. Thus, it is known that all naturally produced PHAs are inherently biodegradable as they can be depolymerized and used by the bacteria that produced them. This does not mean, however, that all PHAs are biodegradable once made into consumer items, because extraction changes their physical (but not chemical) properties.
PHAs are stored in inclusion bodies (what used to be called granules; granules now refer to inorganic storage materials). These inclusions contain the polyester, water and fatty acids which help prevent crystallization of the PHA inside the cell, and proteins which appear to function as scaffolding materials, enzymes for synthesizing the PHA, and depolymerases for breaking it down into monomers. A cell may contain several inclusions. The amount of PHA a cell stores varies with the type of bacterium and the growth conditions. In our hands, Ralstonia eutropha produces around 50% of its dry weight as PHB when incubated with butyric acid in the absence of a nitrogen source. Dr. Kwang-Min Lee, in his Ph.D. studies, was able to get the bacterium to produce 80% of its dry weight as PHB using ice cream as a nutrient source. This same bacterium is used commercially to produce PHBV, and it can be coaxed into making 80-90% of its dry weight as PHBV.
Most bacteria produce PHAs with short sidechains in which the monomers are 4, 5, and 6 carbons long. A group of bacteria of the genus Pseudomonas known as the fluorescent pseudomonads produce only PHAs with long side chains, from 6 carbon monomer units on up. These bacteria produce gummy and elastomeric PHAs from long chain fatty acids as well as from organic solvents such as octane, a well known component of gasoline. Many of these bacteria have an appetite for oil and other petroleum products and may produce these long sidechain PHAs under these conditions of carbon excess.